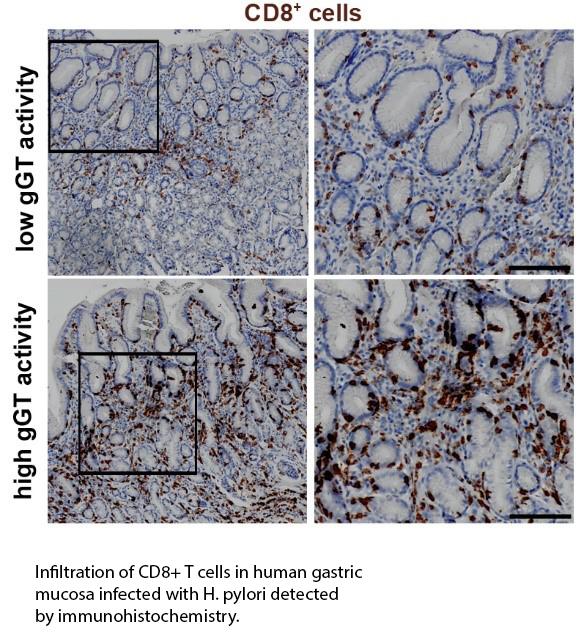
Several virulence factors contribute to H. pylori associated pathology. Among them, H. pylori γ-glutamyltranspeptidase (gGT) is expressed by all H. pylori strains and its activity is correlated with the severity of the disease, emphasizing the central role of this virulence factor in the infection. By catalyzing the hydrolysis of glutamine, gGT participates in bacterial metabolism. Increased levels of ammonia to overcome acidity and a better availability of glutamate used for biosynthesis and energy metabolism are two possible mechanisms by which H. pylori can profit from gGT activity. However, it has not been systematically studied to which extent these mechanisms contribute to growth and survival of H. pylori especially in vivo. Our findings also indicate that gGT is an essential virulence factor driving tolerance and contributing to immune evasion. In dendritic cells, H. pylori gGT influences cytokine secretion skewing the subsequent T cell response towards a regulatory phenotype (Käbisch et al. J Immunol. 2016), while in T cells, H. pylori γ-glutamyltranspeptidase inhibits cMyc and IRF4 expression compromising metabolic adaptation (Wüstner et al. Cell Microbiol. 2015). In vivo, H. pylori gGT is required for the recruitment of CD8+ T cells to the stomach of infected mice, while in humans, gGT activity correlates with CD8 infiltration (Wüstner et al. Sci Rep. 2017). Based on these results, we are now exploring the in vivo function of gGT during colonization and immune response by systematically analyzing the interplay of gGT with the complex stomach environment and the consequences for host cells during H. pylori infection. Our aim is to determine the real contribution of gGT to acid resistance, bacterial metabolism, and immune response during chronic infection. To elucidate the mechanisms by which gGT favors CD8+ T cell recruitment is also a major focus.