

Understanding the immunology of Neglected Tropical Diseases (NTDs) is one of the cornerstones for the development and design of new therapeutic strategies for affected populations. Using samples from human cohorts and from experimental mouse models of infection, and employing novel technologies, we investigate determinants of the host’s immune responses to the causative agents of the most relevant NTDs (e.g., schistosomiasis) to draw on the potential translational aspects in the fight against NTDs.
What are the key features of the NTD immunology?
The immunology of the most clinically relevant NTDs is diverse and complex and depends on the combination of several variables. These include factors related to the environment (region, exposure, humidity, temperature), the parasite (type, stage, anatomical localization, co-infection) and the host (genetic diversity, in utero sensitization, responses). The interaction of these factors generates a complex host-parasite interplay and results in mechanisms that influence the nature of the host immune responses, the course and severity of disease, and the clinical presentations in endemic regions. These mechanisms remain incompletely understood and are one of the primary foci of our research group.

How do helminths survive in their hosts and what can we learn from it?
Helminth parasites co-evolve with their human host and have developed immune regulatory mechanisms whereby they harbor regulatory molecules that induce a wide range of mechanisms, such as Treg and Breg development, to blunt the attack of the host’s immune system. Some of these molecules have been identified as regulators for each phase of the host immune response: initiation, antigen recognition and processing, adaptive and effector cell responses and tissue remodeling and repair. We aim to identify these regulatory molecules, including host homologues (e.g., TGF-ß homolog), mimicry molecules (e.g., TGF-ß-like), lipids and lipid-mediators (e.g., prostaglandins), or extracellular vesicles (exosomes) and understand their mode of action in these regulatory processes. Furthermore, we are exploring their potential applicability in a therapeutic, broadly anti-inflammatory setting of other immune-mediated diseases such as allergies and autoimmune diseases to develop novel treatment approaches.
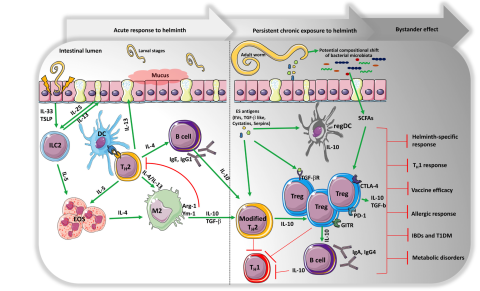
Why do helminths persist in the host for so long?
Helminth parasites co-evolved with human hosts and developed immune regulatory mechanisms whereby parasites harbor regulatory molecules inducing a wide range of mechanisms, such as Treg development, to evade the attack of the host’s immune system, which remains silent. Some of these molecules have been identified and characterized as regulators of every phase of the host immune response: initiation, antigen recognition and processing, adaptive and effector cell responses, and tissue remodeling and repair. The identification of these regulatory molecules, including host homologues (TGF-ß homolog), mimic molecules (TGF-like), TLR-binding, and inhibitors (fatty acid binding protein or anti-inflammatory protein 1/2), or extracellular vesicles (exosomes), and the mechanisms of regulation are under investigation in our group.
What is the One Health approach in NTD immunology?
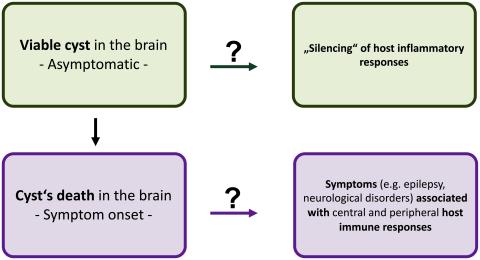
NTDs include not only many parasitic vector-borne diseases but also zoonotic diseases. These zoonoses affect people living in endemic regions, with inadequate sanitation and in close contact with domestic animals and livestock as well as the environment. The One Health approach recognizes that the health of people is closely connected to the health of animals and the environment and is part of the new WHO Road Map 2030 in the fight against NTDs. The development of new therapeutic drugs and vaccines for humans for effective control against NTDs should be also based on understanding the immunology of zoonotic diseases. Within the CYSTINET-Africa Consortium (www.cystinet.org), we are currently collaborating with research partners from Tanzania, Zambia, and Mozambique to elucidate the different immunological characteristics of the zoonotic disease neurocysticercosis, a clinically and radiologically pleomorphic disease of the human central nervous system.
How can a chronic helminth infection have an impact on a vaccine?
Vaccines are one of the most important public health interventions and hence maintaining vaccine efficacy is key to the control and elimination of infections especially in LMIC settings. The evidence from our working group’s animal models demonstrates that co-infection with helminths profoundly affects immune responses to bystander antigens including vaccines. However, clinical studies in humans have so far not yielded a clear picture due to many overlapping helminth, vaccine, and study design factors, and the diverse characteristics of the populations that are studied. Hence, there is the need to systematically review and comprehensively and quantitatively analyze the impact of helminth infections on the immune response to and efficacy of vaccines in humans in order to inform the design of future studies such as investigating the effects of interventions for, e.g., the administration of anti-helminthic treatments before, during, or after vaccination; or the overall impact of underlying helminth infections in adults on novel vaccines such as against malaria and COVID-19 in resource-poor settings.
Current projects in NTDs and Translational Immunology
- The dynamic of macrophage activation during schistosomiasis and reinfection
- Characteristics of the immune responses in HIV patients with Taenia solium (neuro)cysticercosis and during anti-helminthic treatment
- Helminth-derived lipid mediators and extracellular vesicles as modulators of Treg induction and implication for immunotherapy
- Investigating the impact of helminths on vaccine responses and efficacy