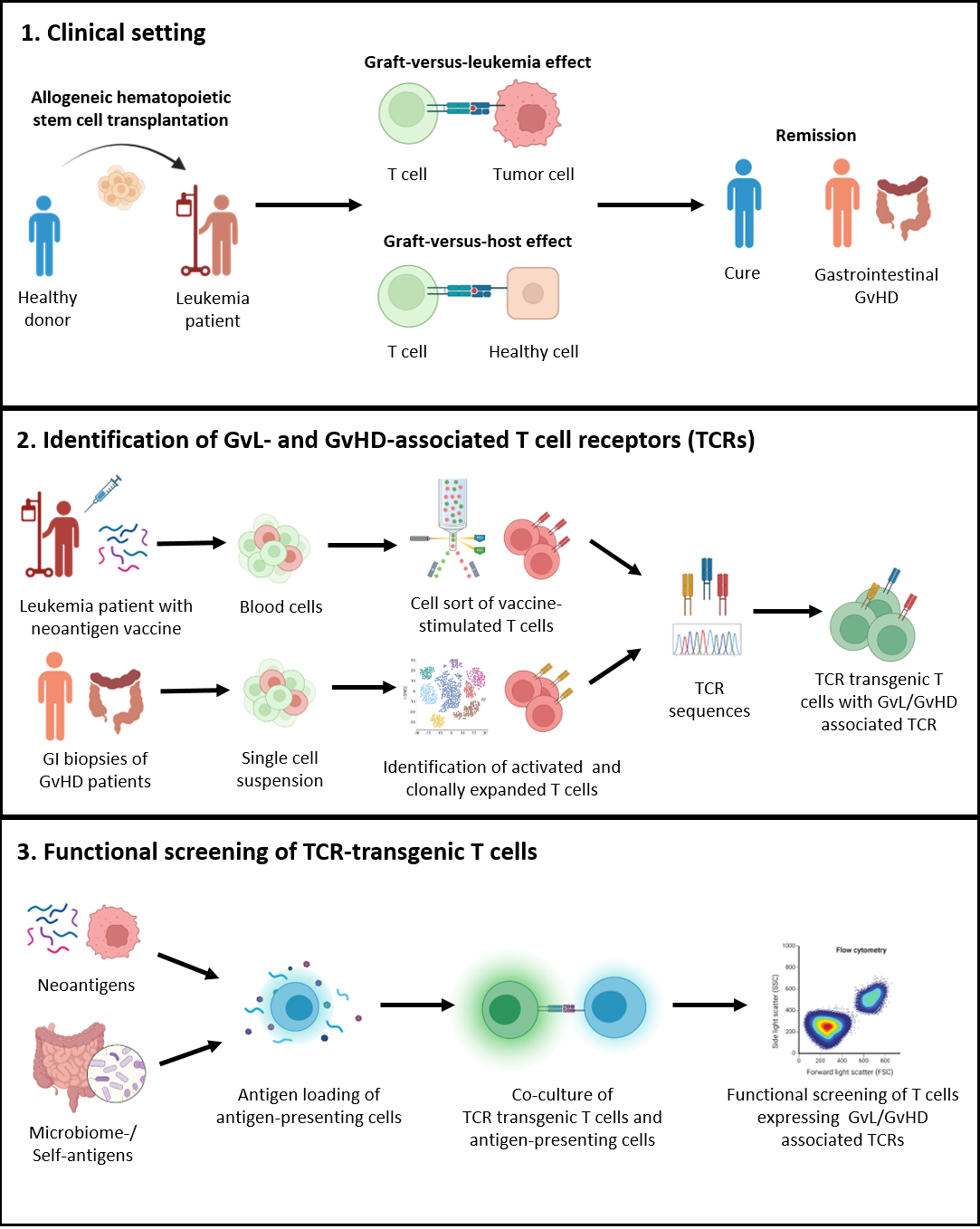
Allogeneic hematopoietic stem cell transplantation (aHSCT) holds promise as a treatment option for hematological malignancies. This procedure involves the removal of a patient's immune system through myeloablative conditioning to eliminate tumor cells, followed by the infusion of healthy donor cells. One of the significant advantages of aHSCT is the graft-versus-leukemia (GvL) effect, wherein the donor cells mount an immune response against any remaining or recurring cancer cells in the patient. However, this approach may lead to complications like graft-versus-host disease (GvHD), where the donor cells also target the recipient's healthy cells. While GvHD has been associated with a reduced risk of leukemic relapses, it can be a life-threatening complication, underscoring the critical need to steer the immune response towards GvL while preventing GvHD.
In pursuit of enhancing leukemia-specific donor cells, novel patient-personalized cancer vaccines have emerged. This approach involves identifying tumor-specific genomic mutations that can be translated into proteins and presented on the patient's tumor cells as neoantigens. These neoantigens are then synthesized and administered as a vaccine. Collaborators in Tübingen have successfully applied this method in treating young leukemia B-cell cancer patients, and observed the emergence of putative vaccine-specific T cells. Our current objective is to identify the T-cell receptors (TCRs) expressed by these cells and analyze their specificity and functionality. This investigation will validate the vaccine's ability to induce a neoantigen-specific T cell response and evaluate whether the quality of this response can strengthen the GvL effect.
Furthermore, recent studies have established a connection between GvHD and the patients' microbiome. Patients with higher microbiota diversity show reduced susceptibility to GvHD, and fecal microbiota transplantation (FMT) has demonstrated success in curing steroid-resistant GvHD. Building upon our prior research utilizing single-cell RNA sequencing and in situ multiplexed tissue imaging via ChipCytometry of gastrointestinal biopsies, we have discovered that severe GvHD patients exhibit lower suppressive capacity of regulatory T cells and a higher fraction of clonal expansion of CD8+ T cells compared to the control group without GvHD. We now aim to investigate the antigens recognized by these putative GvHD-driving T cells and explore the immunomodulatory effects of FMT and conventional GvHD treatment options.
