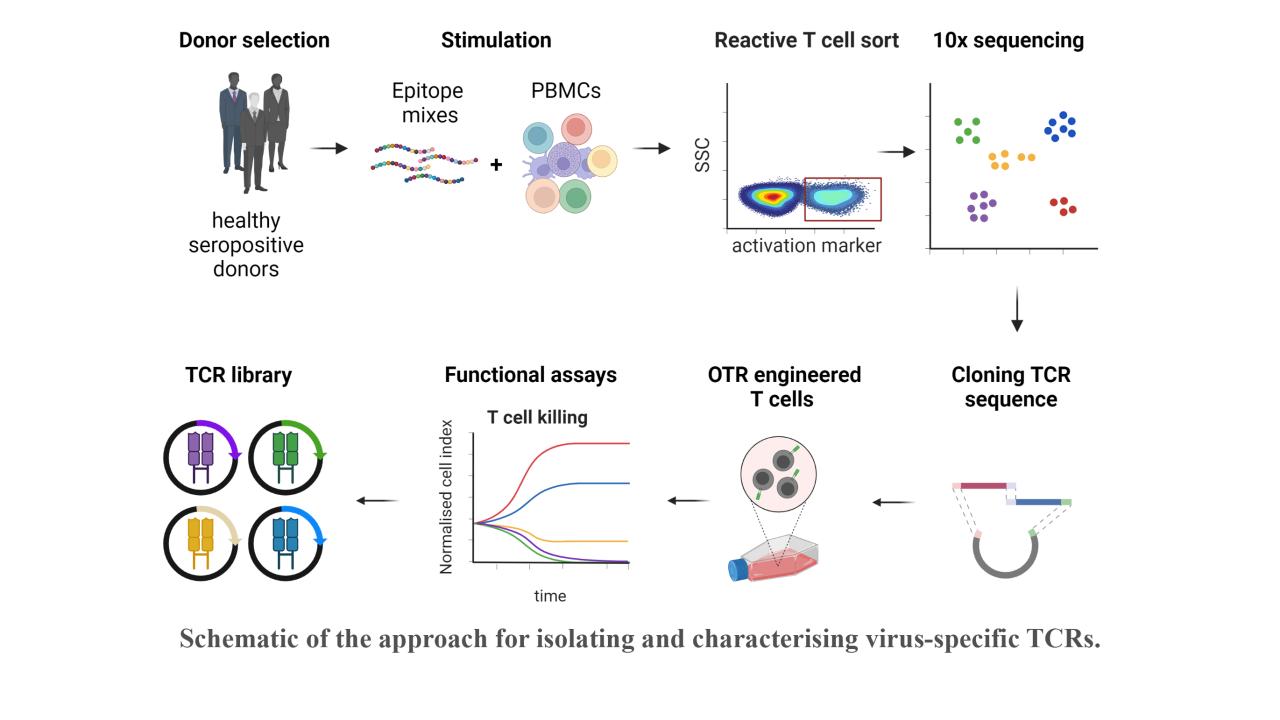
Reactivation of latent viral infections poses a significant threat to immunocompromised individuals, including those who have undergone hematopoietic stem cell transplantation. While antiviral medications are employed to manage these infections, the emergence of antiviral resistance remains problematic. Adoptive T-cell transfer represents a promising solution, specifically for viruses such as human cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus (AdV). The transfer of virus-specific T cells from seropositive donors to immunocompromised patients has shown success in a large number of clinical studies worldwide, including those led by Prof. Busch at the Munich site. However, the isolation of these virus-specific T cells from suitable donors is still technically and logistically complex. A broad applicability can instead be achieved through the use of TCR-engineered T cells. Our current efforts involve identifying immunodominant viral epitopes and their corresponding human leukocyte antigens (HLAs) to render the vast majority of patients eligible for TCR therapy. Subsequently, we utilize these epitopes to stimulate T cells from donors and isolate responsive T cells based on activation markers. Corresponding TCRs are sequenced and subjected to downstream analysis and characterization to select potential candidates for therapy.
With this approach, our goal is to create an "off-the-shelf" TCR library, offering a versatile and accessible solution to effectively combat viral infections in immunocompromised patients.
