



Effective drugs for infectious diseases are core to successful treatment as well as control of pathogen spread on the way to elimination. Almost all pathogens, from bacteria to parasitic helminths, have evolved mechanisms to evade the effects of such drugs, causing drug resistance and treatment failure. Thus, there is a pressing need in the development of new drugs with novel targets. Because the drivers of drug resistance of bacteria as well as of that of many NTDs are manifold, tackling this problem requires an interdisciplinary One Health approach, to include not only human health, but also veterinary and environmental health.
Why are multi drug resistant bacteria one of the most severe threats to human health?
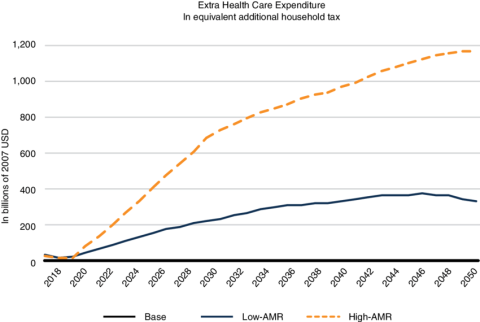
The emergence of multidrug resistant bacteria is a severe threat to our health and healthcare systems worldwide and will be even more so in the near future. Multidrug- (MDR) or extensively drug resistant (XDR) bacteria are resistant to almost every or even all classes of antibiotics that we currently use to fight infections. Antibiotics have become absolutely crucial in modern medicine, and range from post-surgery prophylaxis to treatment of common urinary tract infections. Thus, loosing the armory of effective anti-infective drugs, would throw us back to a pre-antibiotic era with severe implications: it is estimated as many as 10 million people will die annually from multidrug resistant bacteria by 2050, exceeding deaths caused by cancer. In addition, the financial impact would be tremendous and the World Bank currently estimates that the annual economic damage due to MDR/XDR bacteria could become comparable to the 2008/2009 financial crisis, mainly affecting low- and middle income countries (LMIC). An additional 24 million people in these countries would be forced into extreme poverty by 2030. Thus, the fight against AMR must become a global commitment and be part of the AMR roadmaps of the G20 member states as well as many further countries’ AMR roadmaps.

What are drivers of antimicrobial resistance (AMR)?
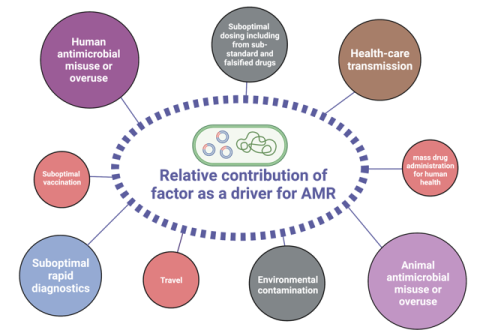
The drivers that are turning AMR into a global threat are manifold. A strong contributor is the overall increase of antibiotic consumption in humans, mainly by false practices such as misuse or unnecessary self-medication, or mass-prescription by physicians. In addition, access to good-quality antibiotics (or the lack thereof) play an important role. In order to prevent resistance, a certain concentration of a drug needs to be reached in the blood and then kept over a defined period of time. Counterfeit antibiotics often tend to have fewer active metabolites, leading to decreased drug levels. Approximately 70% of the consumption of antibiotics deemed medically important for human health are used in livestock mainly for animal husbandry rather than for the treatment of infections, contributing massively to increasing rates of antibiotic resistance. Finally, contamination of the environment constitutes a major problem: subsequent pollution of water bodies by effluents and wastes from pharmaceutical industries or the use of heavy metals in agriculture (e.g., copper as a bactericide or fungicide) contribute to the rise in antimicrobial resistance rates. Interestingly, high concentrations of heavy metals such as arsenic, barium, lead, and zinc have been observed in the environment of areas of prolonged conflict due to heavy artillery projectiles, destroyed infrastructure, and bomb shells. Thus, these are currently being investigated as additional drivers for AMR in our lab.
Why is a ‘One Health’ approach key to tackling AMR and anti-parasitic drug resistance?
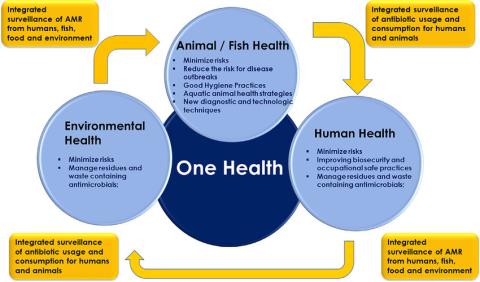
The appreciation of the interconnectedness and interdependence of all living species and the environment is key to human, animal, and planetary health in the future. The One Health approach highlights the synergistic benefit of interdisciplinary and international collaboration in a globalized world. The rapid spread of Covid-19 and the subsequent economic and social shutdown is the latest example of how important this approach is. Evolving drug resistance is another good example of this interconnectedness of our world. As described above, the driving forces of resistance are manifold and diverse. Because pathogens exist almost ubiquitously and bacteria are capable of transferring resistant genes via plasmids, we cannot restrict our approaches to combating drug resistance to human health alone, until we address environmental pollution or the excessive and/or undirected drug use in humans and livestock.
How can we detect drug resistance in parasites or develop novel drugs?
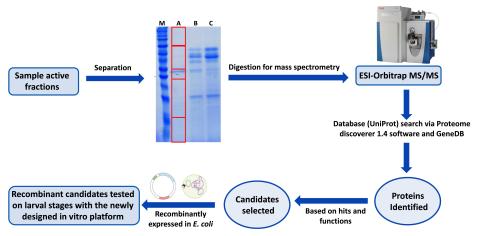
Schistosomiasis, considered the most important NTD, has an enormous impact on public health and economy of developing countries and is targeted by the WHO in its 2021–2030 roadmap for elimination. The only available drug currently used in large-scale mass drug administration against schistosomiasis is praziquantel (PZQ), which solely targets the adult worms but has no effect on early migrating larval stages. However, reinfection is a common observation in endemic regions, and, with the large-scale administration, the development of resistance to PZQ in the field is considered imminent. Thus, the call is out to develop new drugs and/or identify new drug targets. In line with this, we have recently developed a novel and cell-free in vitro platform that allow to identify and test the efficacy of novel host-derived molecules with schistosomicidal activity on various developmental stages (skin, lung, liver) of the parasite. We also utilize our in vitro screening platform in collaboration with industrial partners to identify the targets of their novel compounds using genetic approaches. This characterization is an important part of the next era of Schistosomiasis therapy with an improved understanding of the targets leading to better administration decisions and day 1 monitoring of resistance.
Current projects in AMR and NTD drug development
- Investigating the effect of heavy metals on carbapenem resistance in Acinetobacter baumannii
- Tackling antimicrobial resistance among refugees and internally displaced communities by improving access to quality antibiotics
- Characterization of host-derived schistosomicidal molecules for novel drug development